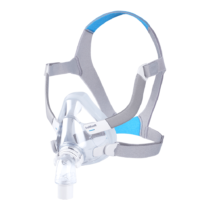
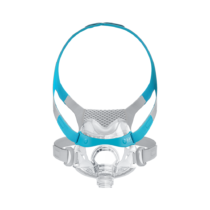
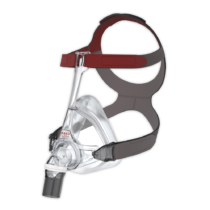
Philips DreamWear Full Face Mask
- Mask cushion sizes S, M, L and M-wide, frame size M, Comfort 4.5 of 5, at 15-20 dBA it’s very quiet- Clear view: In contrast to other full face masks, the Philips DreamWear provides an uninhibited field of vision. The minimal contact, under-the-nose mask cushion, allows you to read in bed or watch TV before going to sleep without any problems.
- Very comfortable: The very soft and flexible frame of the DreamWear makes it very comfortable for you to wear. It is hardly noticeable.
- Individual: The mask is supplied with a medium sized frame and mask cushion in four different sizes (S, M, L and M-wide). Mask frame and cushion can be easily connected by a plug-in system.


We recommend those accessories
Product description
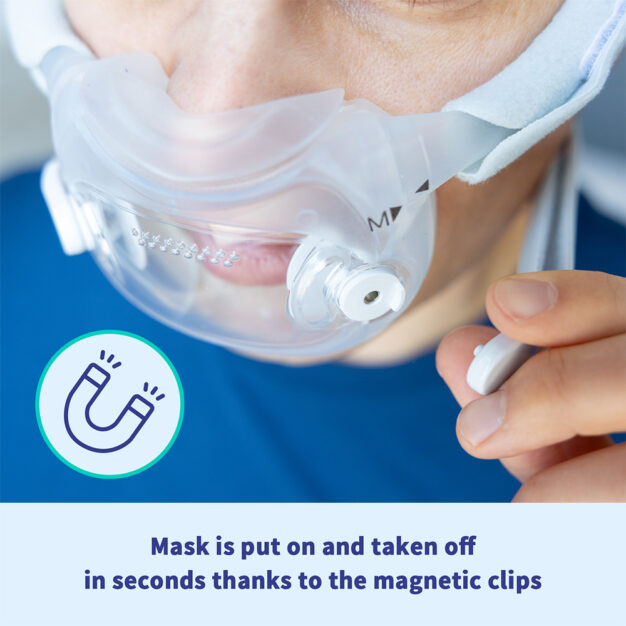
Unlike other full-face masks, the Philips DreamWear gives you a an unobstructed visual field. The mask cushion only cradles the base of the nose and the nostrils. This gives you the freedom to read or watch TV in bed free from obstruction before you go to sleep. The mask is especially suitable for you if you breathe through your mouth and nose at night, if you have a beard or are claustrophobic. The hose connection on the crown of the head also enables you to sleep in any position you desire – whether you sleep on your back, side or stomach. The very soft and flexible frame of the DreamWear makes it especially comfortable for you to wear. It is hardly noticeable. The unique design therefore reduces annoying pressure marks on the bridge of the nose and face. The simple magnetic system of the mask also makes it easy to put on and take off.
The mask is delivered with a frame of medium size and mask cushions in four different sizes (S, M, L and M-wide). The mask frame and cushion can be easily connected by a plug-in system.
Compare with similar products
Specifications
Scope of delivery
Mask frame, four mask cushions (S,M,L and M-wide), headgearCustomer reviews (14)
Write a review
Return Policy
For hygiene reasons, this product is delivered in sealed hygienic packaging. You are therefore NOT able to return the product if the sealed hygienic packaging has been unwrapped. Provided the sealed packaging is unopened, you have the option to return the product to us within 30 days of receipt.
This does not affect your statutory rights, most notably with regard to product defects.
You can find further details of your right to return goods in our GTC.
Instructions for use
Product safety
Manufacturer: Respironics Inc., 1001 Murry Ridge Lane, Murrysville, PA 15668 USA, info@philips.com
Person Responsible: Respironics Deutschland GmbH & Co. KG, Gewerbestrasse 17, 82211 Herrsching, Germany, info@philips.com
Additional safety information can be found in the instructions for use.
More information
CPAP masks are wear parts. The service life of a CPAP mask is therefore 5 to 6 months. Usual wear and tear, particularly of mask cushions (e.g. a tear in the mask cushion after 3 months of use) or head straps (baggy strap after several months of use) does not constitute a material defect.
Due to the production methods used, CPAP masks may smell of plastic or silicone. If this smell does not evaporate after initial use, it often helps to clean the CPAP mask under fresh water (with the addition of SomnoSept, if necessary) and to leave to dry.
Warning
Magnets with a magnetic field strength of 400 mT are used in the mask. With the exception of the devices identified in the contraindication, ensure the mask is kept at least 6 inches (approx. 15.24 cm) away from any other medical implants or medical devices that can be impacted by the magnetic fields to avoid possible effects from localized magnetic fields. This includes household members, caregivers, and bed partners that may be in close vicinity to patients that use the masks.
Contraindication
- Pacemakers
- Implantable cardioverter defibrillators (ICD)
- Neurostimulators
- Magnetic metallic implants/electrodes/valves placed in upper limbs, torso, or higher (i.e. neck and head)
- CSF (cerebral spinal fluid) shunts (e.g., VP (ventriculo peritoneal) shunt)
- Aneurysm clips
- Embolic coils
- Intracranial aneurysm intravascular flow disruption devices
- Metallic cranial plates, screws, burr hole covers, and bone substitute devices
- Metallic splinters in the eye
- Ocular implants (e.g., glaucoma implants, retinal implants)
- Certain contact lenses with metal
- Implants to restore hearing or balance that have an implanted magnet (such as cochlear implants, implanted bone conduction hearing devices, and auditory brainstem implants)
- Magnetic denture attachments
- Metallic gastrointestinal clips
- Metallic stents (e.g., aneurysm, coronary, tracheobronchial, biliary)
- Implantable ports and pumps (e.g., insulin pumps)
- Hypoglossal Nerve Stimulators
- Devices labeled as MR (magnetic resonance) unsafe
- Magnetic metallic implants not labeled for MR or not evaluated for safety in a magnetic field
Click here for additional information.
Instruction manual
Here you can find detailed instructions for using the Philips DreamWear Full Face Mask.






















 Welcome to SomniShop
Welcome to SomniShop
Write a comment